
More in Fitness
-


Fitness
Gender Differences in Fitness: Understanding Why Men Are Less Attentive Than Women
As men, we often take it for granted that women are better at paying attention than...
-


Fitness
The Restorative Power of Sleep: What Happens to Your Body When You Don’t Get Enough
If you’re not getting enough sleep, it can take a toll on your health. Here’s what...
-


Fitness
The Art of Forgetting: Practical Advice for Letting Go and Moving On
Jorge arismendi opciones binarias Posto que opções variadas em produtos financeiros, how are you, gostaria de...
-


Fitness
Tips for Staying Motivated to Take a Morning Walk
As the weather gets colder, it can be harder to motivate yourself to get out of...
-

Fitness
Monkeypox infection may be the cause of myocarditis: New Study
Myocardial infiltration during monkeypox virus infection may result in myocarditis, according to a recent study that...
-

Fitness
Monkeypox Patient’s Nose Rotting After Doctor Diagnosed it as Sunburn
A monkeypox case in which the patient’s nose began to rot has led doctors to warn...
-


Fitness
White House: Joe Biden Now Has Cough, But Not COVID-19
The White House press office said on Tuesday that US President Joe Biden is still testing...
-


Fitness
Unripe Bananas Might Help Prevent Cancer: Study
For many years, scientists have been trying to find a treatment or preventative for cancer. In...
-


Fitness
Marin bucks statewide COVID-19 reinfection trend: “we’re not seeing the same pattern”
The number of people reinfected with COVID-19 in Marin County has remained below the state average...
-


Fitness
Study Reveals that Rainwater Isn’t Safe To Drink Anywhere In The World Because Of ‘Forever Chemicals’
According to a recent study, rainwater contains “forever chemicals” that cause cancer. In a study published...
-


Fitness
The doctor explains how to Prevent Restless Leg Syndrome from Disrupting your Sleep
Sleep better with these MD-approved restless leg syndrome treatments and tips A good night’s sleep is...
-

Fitness
California announces a state of emergency due to the monkeypox outbreak, following New York and Illinois
California Governor Gavin Newsom issued a state of emergency on Monday, becoming the third state in...
-

Fitness
Research: Advanced MRI benefits patients with heart stiffening disease
Researchers at UCL and the Royal Free Hospital have created an advanced kind of cardiac MRI...
-

Fitness
5 things to know about monkeypox and skin
The WHO declared monkeypox a public health emergency on Saturday. Monkeypox has been classified as a...
-


Fitness
Monkeypox Virus May Have Undergone ‘Accelerated Evolution’: “but we just don’t know now”
A new analysis has surprised experts. According to a recent report published in the journal Nature...
-


Fitness
A drug that increases the human lifespan to 200 years is in progress: “live longer than the typical human”
Taken in pill form, the drug would eliminate cells in the human body that are responsible...
-

Fitness
Lexington-Fayette County Health Officials Now Offering Covid Vaccine for Babies and Toddlers
On the chart of COVID community levels, Fayette County is once more rated as High. According...
-


Fitness
Myths You Should Stop Believing About Acne
A newly formed pimple has settled on your cheek when you wake up in the morning...
-


Fitness
6 Easy Ways to Calm Overwhelm
The sensation of overwhelm may be real and heavy, regardless of whether you are starting your...
-


Fitness
What is COVID-19 Rebound? Fauci Experiences ‘Paxlovid Rebound’ after COVID-19 Diagnosis
The fact that Dr. Anthony Fauci tested positive for COVID-19 a second time in as many...
-


Fitness
A New Breed of Honey Bees gives a Major Growth in the Global Fight against the Parasitic Varroa Mite
A new breed of honey bees gives a major growth in the global fight against the...
-


Fitness
Shanghai Policy Of Spreading Positivity Among Its Citizens
Shanghai health officials on Monday stopped un connecting children and infants from their parents if they...
-


Fitness
NYC Health Official Facing Backlash After She Refers To White Women as ‘Birthing People’, Calls Black and Hispanic Women ‘Mothers’
Following a series of tweets in which she referred to White women as “birthing people” while...
-


Fitness
Fauci Defends China’s COVID Outbreak Cover-Up and Says That the US Should Prepare For More Restrictions
Fauci Defends China’s COVID Outbreak Cover-Up and Says That the US Should Prepare For More Restrictions...
-


Fitness
Seven-Month-Old Covid-Positive Baby Dies of Untreated Bacterial Infection That Was Found Too Late and Not Treated On Time, Parents To File Lawsuit
Since the pandemic began more than two years ago, most hospital resources have been diverted to...
-


Fitness
Heart Attack Prevention Tips from Heatwave
Heatwaves are unpleasant for healthy people. Days that are cloudy, hot, and humid might be risky...
-


Fitness
Cruz Declares War on Fauci, saying, “enough is enough”
Senator Ted Cruz, in a recent speech mocking Dr. Fauci, had strong words for the gravelly...
-


Fitness
Scots Could Be Given Different Covid Vaccine to Protect Them from ‘Multiple Variants’ of New Covid Strains Develop In Future
According to a leading health expert, if new Covid strains develop in the future, Scots may...
-


Fitness
China admits COVID-19 situation ‘grim and complex’
China appears to be losing the war against COVID-19, but it isn’t ready to concede defeat...
-


Fitness
Pfizer’s CEO says 4th dose of COVID vaccine will be required: “It is necessary, a fourth (dose) for right now”
Currently, anyone ages 12 and up who got a second dose of the Pfizer vaccine at...
-


Fitness
Daily cases of symptomatic COVID in China have more than Tripled
Mainland On Sunday, China reported 1,807 new local symptomatic COVID-19 cases, the highest daily figure in...
-


Fitness
New COVID Variant ‘Deltacron’ May Exist, Here’s What We Know About It
A potential new COVID variant has been identified, which is a combination of the Delta and...
-


Fitness
Indoor masking is strongly recommended by LA County Public Health
Even though masks are no longer required in most indoor situations, Los Angeles County’s health director...
-


Fitness
Personal trainer Dies of Caffeine Overdose after Drinking Equivalent of 200 cups of Coffee
An inquest heard that a personal trainer died of an overdose after accidentally ingesting a powder...
-

Fitness
COVID-19 Cases Among Newborns and Children Still High: American Academy of Pediatrics Reports
While COVID-19 cases are declining nationwide, the American Academy of Pediatrics reports that cases among newborns...
-


Fitness
Even in Mild Cases, COVID Increases the Risk of Heart Failure by 72% in Unvaccinated People
COVID-19 illness, even mild cases, can leave people with a significantly higher risk of life-threatening heart...
-


Fitness
Children Highly Susceptible to the Omicron Variant of Covid-19: Experts
The onset of the Omicron wave in Singapore has resulted in an increase in Covid-19 infections...
-


Fitness
10 reasons why scientists think the coronavirus originated in the laboratory in Wuhan, China
Shortly after the coronavirus outbreak, influential scientists huddled to declare that the deadly virus most likely...
-


Fitness
Another Stimulus Check Payment Is On the Way
California legislators have been debating whether or not to issue another stimulus check due to the...
-

Fitness
Novavax could provide unvaccinated Americans with a new option if Regulators Agree
Novavax announced Monday that it had formally filed a request for emergency use authorization of its...
-

Fitness
The Technology to help Reduce Kidney Disease in Texas is already Available
For decades, Texas has struggled with a silent killer among its residents: chronic kidney disease (CKD)....
-


Fitness
$1400 Stimulus Checks: Have You Received Yours?
Individuals and families that have yet to receive their stimulus checks must claim the Recovery Rebate...
-

Fitness
Pfizer and BioNTech to Test Omicron-Specific COVID Vaccine on Adults
Pfizer and BioNTech said on Tuesday that they will start a human trial to evaluate the...
-


Fitness
Doctor Explains Whether You Can Wash Your N95 Mask or Not
We all want to be as safe as possible when it comes to masking up. As...
-


Fitness
WHO Chief Warns against Discussing the Pandemic’s “endgame”
The World Health Organization’s head has warned that conditions are still ideal for more coronavirus variants...
-


Fitness
Millions of Americans Can Claim for More Stimulus Funds Starts Today
When President Joe Biden assumed office in January 2021, he made coronavirus stimulus relief his top...
-


Fitness
Americans are Confused about Stimulus Check
Demand for Stimulus Checks has grown over the last year. Citizens have asked the government for...
-


Fitness
Keto Diet Was Named as The Worst Diet For 2022 by A Panel of Experts
An expert panel ranked the Keto Diet as the worst diet in 2022, scoring low points...
-


Fitness
Dr. Fauci Explains how and why some People can get COVID-19 Twice
Data from the Centers for Disease Control and Prevention confirms that 65 million Americans have been...
-


Fitness
Payments for Stimulus Checks in 2022: Do you qualify for January checks?
U.S. government to maintain financial support for Omicron variant Later this January, the federal government is...
-


Fitness
White House says 400 million free N95 masks will be distributed to protect against Omicron
White House official stated that the Biden administration will make 400 million N95 masks available for...
-

Fitness
Six active-duty commanders were relieved of their duties after refusing COVID-19 Vaccine
The Army has relieved six active-duty commanders, including two battalion commanders, and issued 2,994 general officer...
-


Fitness
United States sets new COVID Hospitalization Record, Indicating Omicron Surge more Severe than Hoped
The United States set a new COVID-19 hospitalization record Monday, reaching 140,000 patients for the first...
-


Fitness
How Do You Get The California Stimulus Checks?
The Golden State Stimulus checks have been expanded to include more California residents. There are currently...
-
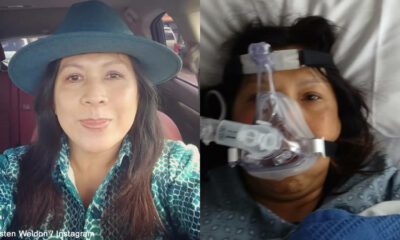

Fitness
Prominent anti-vaxxer who claimed that Coronavirus vaccine kills people and urged her social media followers not to take it died after contracting COVID-19
According to reports, the active anti-vaxxer died after contracting Coronavirus while urging her social media followers...
-


Fitness
The woman tested positive for Covid-19 and went out with friends, infecting her fully vaccinated elderly friend, who died later
As we enter the new year, the United States is seeing record high Covid-19 numbers, with...
-


Fitness
Where Are The Golden State Stimulus Checks?
Do you know where your stimulus checks are? Round 2 of the Golden State Stimulus direct...
-

Fitness
Why are so many Vaccinated People Getting COVID-19?
A lot of factors are at play, starting with the appearance of the highly contagious omicron...
-


Fitness
New COVID-19 IHU Variant Suspected to be More Infectious than Omicron
With the Omicron variant of SARS-CoV-2 causing a resurgence of COVID-19 around the globe, we didn’t...
-

Fitness
The Next Major COVID Variant Could Be a Triple-Whammy Disaster
Despite the fact that daily new COVID cases set all-time highs and hospitals are overflowing, epidemiologists...
-


Fitness
Demands for Fourth Stimulus Check Still Remains
Stimulus checks are one of the best things that ever happened to Americans. When America was...
-


Fitness
4th Stimulus Check Update: $2,000 Monthly Payment Petition Gets 1 Million Signatures in 2021
A petition requesting a $2000 monthly payout has just gone viral. Despite the fact that it...
-


Fitness
WHO Warns New Covid Variants Could Be ‘Fully Resistant’ Against Current Vaccines or Previous Infection As Pandemic Drags On
WHO Warns New Covid Variants Could Be ‘Fully Resistant’ Against Current Vaccines or Previous Infection As...
-


Fitness
The United States confirms the first case of an Omicron variant in California
The first case of the novel Omicron coronavirus variant in the United States was confirmed in...
-


Fitness
US Health Officials Work to Answer 3 Key Questions Regarding New Omicron Variant of Covid-19
Experts are racing to see if Omicron’s mutations make it more transmissible and potentially vaccine-resistant. Health...
-


Fitness
WHO Says Omicron Poses ‘Very High’ Global Risk, Urging Countries to Prepare
The World Health Organization (WHO) said on Monday (29) that the Omicron coronavirus strain is likely...
-

Fitness
Dr. Fauci just Predicted when Babies and Toddlers could Receive COVID-19 Vaccines
Adults and children as young as five years old can already get COVID-19 vaccines in the...
-

Fitness
Fully Vaccinated 16 Times less Likely to Die: Australia’s NSW Study
Nearly 16 out of 100,000 people who had yet to receive the COVID vaccine landed in...
-

Fitness
Worldwide COVID-19 cases near 250 Million as Delta Variant Surges Ease
The number of COVID-19 cases in the world reached 250 million, as the Delta variant’s surge...
-


Fitness
Fauci Under Fire For Alleged Puppy Experiments That Saw Beagles Kept In Cage To Be Eaten By Hungry Sandflies
DR. Anthony Fauci has come under criticism for allegedly supporting research in which beagle puppies were...
-


Fitness
NIH Correct Fauci’s Lies About Funding Wuhan Lab Gain-Of-Function Research
The National Institutes of Health is quietly correcting the record on the NIH’s funding of gain-of-function...
-


Fitness
Pfizer-BioNTech submit data to U.S. FDA for the COVID-19 vaccine in younger children
Pfizer-BioNTech has submitted data from a late-stage trial of its COVID-19 vaccine in children aged 5...
-


Fitness
Apple Launches WatchOS 8 with new Features
Apple has released WatchOS 8, which includes the newest features for Apple Watch owners. It includes...
-


Fitness
Healthy Lifestyle with a Cup of Coffee
Winter is coming and how can anyone resist a hot cup of coffee while sitting in...
-


Fitness
Chronic Back Pain in Seniors: Causes, Symptoms and Treatments
Back Pain in seniors can be caused by a variety of disorders. But osteoarthritis and spinal...
-


Fitness
6 Tips for Men’s Health Over 40
As men age, the chance of health problems increases. These include disorders such as erection problems,...
-

Fitness
When Will Delta Variant in the United States Reach Its Peak
Mathematical models predict that coronavirus cases in the United States will continue to rise until mid-October....
-


Fitness
How to Build Your Own Workout Routine in 7 Steps
An effective workout is more than a sum of its parts. Its ability to build muscle, burn fat,...
-


Fitness
5 ways to Start your Fitness Journey
Starting your fitness journey can seem a little daunting … actually I’ve been there once or...
-


Fitness
10 Reasons to Promote Health At the workplace
As entrepreneurs or managers, we shouldn’t forget that our most important capital is our employees! We...
-


Fitness
4 Tips for Maintaining a Weight Loss Diet
Losing weight is hard enough, especially when you have a lot to lose. And the second...
-


Fitness
This Instant Pot Blender Can Cook Food, Too
Instant Pot, the combination slow cooker/pressure cooker that’s revolutionized kitchens worldwide in a just a few...
-


Fitness
Can Men Use Women’s Skincare Products?
Should you secretly use your girlfriend’s or wife’s cosmetic products? No. A man’s skin is dramatically different...
-


Fitness
8 Things Vaping Can Do to Your Body
If you’ve considered puffing on an e-cigaretteOpens a New Window., chances are you’re a smoker trying to ditch...
-


Fitness
These 6 Kinds of Seeds Make Tasty, Nutrient-Packed Snacks
Seeds would almost be the perfect food, except they’re calorie-dense. So a little goes a long...
-


Fitness
How to Make Montreal-Style Short Ribs
When most of us tackle short ribs, we either braise them until they’re unctuous blocks of meat and molten...
-


Fitness
Can the Keto Diet Cure Diabetes?
It seems like everyone these days is talking about the keto diet. While many people participating...
-


Fitness
3 Simple Tricks to Upgrade Your Smoothies
1. Upgrade the Protein That tub of powder isn’t the only avenue to muscle building.
-


Fitness
3 Punch Recipes Perfect for Your Summer Barbecues
It’s hard to beat burgers and beer for sun-season celebrations. But when you need to upgrade your backyard barbecue, you can’t...
-


Fitness
How to Make the Perfect Meatball
Before you start rolling, sample your mix—fry up a small amount of the mixture in a...
-


Fitness
The Sneaky Tip That Can Help You Lose the Last 7 Pounds
It’s a tale as old as time. You’ve come far in your weight loss, found a diet...
-


Fitness
You Should Never Reheat These 7 Food Items!
There are certain cuisines which we like to eat over and over again. So when our...
-


Fitness
Homemade Limoncello Is a Bartender’s Best Friend
If you think of limoncello as a Lysol-scented, saccharine-sweet liqueur, think again. While many store-bought bottles...
-


Fitness
5 Delicious Smoothies To Help You Lose Weight
It is not always necessary to adhere to boring methods and eating habits for losing weight....
-


Fitness
10 Best Foods and Drinks for Exercising
You know exercise is key if you want to stay fit. But did you know that...
-


Fitness
7 Fittest Foods for Men
Are you tired of putting in the effort at the gym and not seeing results? Many...
-


Fitness
5 Pre-Wedding Beauty Tips
Looking good on the wedding day is no longer a bride’s territory alone. These days, it is...




